Kidney International - Expression of apoptosi...
来源:百度文库 编辑:神马文学网 时间:2024/05/01 10:42:51
- Jump to main content
- Jump to navigation
- nature.com homepage
- Publications A-Z index
- Browse by subject
- ISN
- My account
- Submit manuscript
- Register
- Subscribe
Login
Cell Biology – Immunology – Pathology
Kidney International (2000) 57, 969–981; doi:10.1046/j.1523-1755.2000.00925.x
Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure
Alberto Ortiz, Corina Lorz, Marina P Catalán, Theodore M Danoff, Yasushi Yamasaki, Jesús Egido and Eric G Neilson
Fundación Jiménez Díaz and Universidad Autónoma de Madrid, Madrid, Spain; Penn Center for Molecular Studies of Kidney Diseases, Renal-Electrolyte and Hypertension Division of the Department of Medicine, and the Graduate Groups in Immunology and Cell Biology, University of Pennsylvania, Philadelphia, Pennsylvania; and Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA
Correspondence: Eric G. Neilson, M.D., Department of Medicine, D-3100 MCN, Vanderbilt University Medical Center, Nashville, Tennessee 37232-2358, USA. E-mail: eric.neilson@mcmail.Vanderbilt.edu
Received 1 June 1999; Revised 28 September 1999; Accepted 4 October 1999.
Top of pageAbstract
Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure.
Background
While tubular cell death is a characteristic of acute renal failure (ARF), the molecular mechanisms that modulate this cell death are unclear. Cell fate in acute renal failure hinges on a balance of survival and mortality factors in a changing environment. We further explored this issue by studying selected cell death-related proteins in experimental renal failure.
Method
The expression of genes that promote (c-myc, Bax, BclxS) or protect (Bcl2, BclxL) from cell death was studied by Northern blot, Western blot, and immunohistochemistry in murine kidneys following ARF induced by folic acid or in renal tubular epithelial cells (MCT) stressed in culture.
Results
Renal mRNA levels encoding for c-myc and BclxL were elevated in ARF while the Bcl2/Bax ratio was decreased (Bcl2 decreased and Bax increased; P < 0.05). Protein levels of BclxL increased and Bcl2 protein decreased. Expression of tumor necrosis factor (TNF-), a mediator of ARF, was also increased. Immunohistochemistry further demonstrated that BclxL was increased in some tubuli and absent in others, while Bcl2 expression decreased diffusely. Bax staining was also patchy among tubuli and individual cells in the tubular wall and lumen. As a relative deficit of survival factors is present in ARF, MCT epithelium were deprived of serum survival factors. This resulted in apoptosis, decreased Bcl2/Bax and BclxL/Bax ratios (P < 0.05) and sensitization to TNF-
-induced apoptosis (P < 0.05). The latter was prevented by enforced overexpression of BclxL (P < 0.01). TNF-
increased the mRNA levels encoding for c-myc and decreased BclxL expression. Neither MCT cells nor the kidney expressed BclxS.
Conclusions
A relative deficit of survival factors likely contributes to changes in levels of BclxL and Bax in ARF. These deficits predispose to cell death induced by persistent lethal factors such as TNF- that is increased in ARF and a potential source of increased c-myc, a downstream facilitator of cell death. These findings implicate members of the Bcl2 family of proteins as regulators of tubular cell death in ARF and single them out as potential therapeutic targets.
Keywords:
apoptosis, Bcl2, Bax, BclxL, acute renal failure, kidney, tubular cells, TNF
Acute renal failure (ARF) is often associated with renal tubular cell death. In experimental models of ARF cell death is partly mediated by apoptosis1. Apoptotic cells have also been demonstrated in human kidneys with ARF2. In addition, apoptotic tubular cell death may facilitate the tubular atrophy of chronic renal injury3. At present, however, there is little information on the factors that promote or regulate apoptosis in kidney.
The balance between factors that contribute to survival growth or lethality often impacts on the chance of cell death4,5. For example, competition for limiting amounts of survival factors results in the apoptotic loss of 50% of newly formed oligodendrocytes during central nervous system development6. A similar balance may play a role in determining the extent of cell death in ARF. In fact, there is evidence that a relative deficit of survival factors contributes to ARF. Multifunctional cytokines such as insulin-like growth factor (IGF-1) and endothelial growth factor (EGF) have survival factor activity for tubular epithelium. The local expression of cytokines with survival factor activity is decreased in ARF7,8,9,10 and the cells compete for these cytokines by increasing the expression of receptors7,10. The exogenous administration of cytokines with survival factor activity improves the evolution of ARF and reduces the amount of apoptosis11,12. However, the molecular mode of action of survival factors is not well understood.
Other multifunctional cytokines such as tumor necrosis factor- (TNF-
) also promote apoptosis and induce acute tubular necrosis13,14, while anti-TNF-
antibodies protect against renal failure in model systems15. Although not conclusive, these findings are consistent with the hypothesis that changes in the balance between factors with survival and mordant activity in tubular epithelium may modulate cell death genes in ARF, and thus determine cell fate.
Some apoptosis regulatory proteins relevant to renal pathology have been characterized. These include members of the Bcl2 family of proteins (Bcl2, Bax, Bclx, and others) and the transcription factor c-myc16,17,18,19. The main known function of Bcl2 family members is to regulate cell death. Bcl2 is an intracellular membrane-associated protein whose expression prevents or delays apoptotic cell death in response to a number of stimuli, including deprivation of survival factors and, in some cells, activation of TNF- receptors19. Bcl2 also protects against cell death where there are morphological features of necrosis such as death induced by buthionine sulfoximine in neural cells20. In this regard the same stimulus, depending on the intensity, may induce either apoptosis or necrosis. The protective effect of Bcl2 is also evident in a model of ischemic cell death in which both modes of cell death have been identified: Bcl2 overexpression in the brains of transgenic mice decreased experimental infarct size 50%21.
Bclx, a member of the Bcl2 family, has two alternatively spliced forms: BclxL and BclxS. BclxL, like Bcl2, protects cells from a wide variety of apoptotic stimuli16. In contrast, BclxS antagonizes the protective effect of Bcl2 and BclxL16. Bax is a Bcl2-like protein that binds to and antagonizes the protective effect of Bcl2 and BclxL, rendering cells more sensitive to death18. In this sense, the ratio of expression of Bcl2 or BclxL to Bax or BclxS appears to determine cell fate in an adverse microenvironment18.
Finally, c-myc favors cell death when the microenvironment is adverse for survival. Overexpression of c-myc can make cells more dependent on external growth factors for the prevention of apoptosis17. This growth factor dependence can be overcome by overexpression of protective proteins such as Bcl222.
To better understand the determinants of renal cell death, we examined the expression of the aforementioned genes under two conditions. In vivo, we examined their expression in a model of murine ARF. In vitro, we examined their expression in cultured tubular epithelium which were forced into an apoptotic program by serum deprivation or by TNF- stimulation.
METHODS
Animals and cell culture
SJL mice were obtained from the Jackson Laboratories (Bar Harbor, ME, USA). Folic acid nephropathy was induced in 5- to 7-week-old mice by a single intraperitoneal injection of folic acid (Sigma, St. Louis, MO, USA) 250 mg/kg in 0.3 mol/L sodium bicarbonate23. Three mice were killed at different time points (from 6 h to 7 days) after injection of either folic acid or vehicle (0.3 mol/L sodium bicarbonate). Six additional mice were killed at 24 hours. The kidneys were saline perfused. One kidney from each mouse was fixed in buffered formalin, included in paraffin and stained with hematoxylin-eosin or used for immunohistochemistry. The other was snap-frozen in liquid nitrogen for RNA, DNA and protein studies. Blood was drawn from the heart and serum creatinine was determined at 24 hours.
MCT cells are a cultured line of proximal tubular cells harvested originally from the renal cortex of SJL mice24. The cells were maintained in culture in DMEM supplemented with penicillin 100 /mL and streptomycin 100
g/mL and 10% heat-inactivated fetal calf serum (FCS; DMEM-10%) as previously described24. A human BclxL cDNA (a generous gift from Craig B. Thompson, University of Chicago) was cloned into a vector which contained a 1.2 kb fragment of rat gamma glutamyl transferase promoter and the human GH polyadenylation signal. This vector directs expression in tubular cells25. MCT cell lines constitutively expressing human BclxL or an empty control vector were established. For that purpose MCT cells were electroporated at 960
F and 220 V and cultured in the presence of 600
g/mL G418 for three weeks. An increased expression of BclxL was confirmed in MCT-BclxL by Western blot.
For those experiments in which the effect of serum deprivation or TNF- stimulation was examined, the cells were plated and then grown for 24 hours in DMEM-10%. Then the media was replaced with fresh DMEM-10% or serum-free DMEM (DMEM-0%). The DMEM-0% contained the same supplements as the serum-supplemented DMEM with the exception of the FCS.
Murine TNF- (60 U/ng; Boehringer Mannheim GmbH, Mannheim, Germany) was added to some cultures to achieve a final concentration of 30 ng/mL. When TNF-
was added to serum-free cultures, the cultures were grown for 24 hours in DMEM-0% prior to the addition of the TNF-
. Preliminary experiments had demonstrated that this concentration of TNF-
is cytotoxic.
Assessment of cell death/apoptosis
For quantification of cell death, 10,000 cells were seeded in 24-well plates (Corning Costar, Cambridge, MA, USA) in DMEM-10%, and after 24 hours the culture conditions were modified as described above. Thereafter at defined time points, the cells were harvested from the wells. The nonadherent cells were pooled with the adherent cells, which were detached from the plate by gentle trypsinization. The total number of cells were counted and the cell viability was determined by trypan blue exclusion. For this purpose at least 100 cells from triplicate wells were counted in a Fuch-Rosenthal chamber.
Apoptosis was also assessed by flow cytometry26,27. Cells attached to the plate were collected and mixed with detached cells present in the supernatant. Cells were spun and resuspended in 100 g/mL propidium iodide, 10
g/mL RNAse A, 0.05% NP-40 in phosphate-buffered saline (PBS), incubated at 4°C for one hour, and analyzed on the FACScan using LYSIS II software. The percentage of hypodiploid cells, as assessed by decreased DNA staining (A0), comprising apoptotic cells with fragmented nuclei, was counted. For comparisons between cells overexpressing BclxL and control cells, the percentage of specific cell death was quantified. For that purpose, cell death in the absence of TNF-
was subtracted from cell death in the presence of TNF-
. Cell death thus calculated in control cells was considered to be 100%.
To assess for the pyknotic nuclear changes seen in apoptosis, cells were stained with propidium iodide. MCT cells were plated onto Labtek™ slides (Nunc Inc., Napersville, IL, USA) in DMEM-10%. After 24 hours the media was changed to either fresh DMEM-10% or DMEM-0% and then grown for an additional 48 hours. The cells were stained with propidium iodide basically as previously described28. Briefly, the slides were washed with PBS, fixed for 10 minutes in 10% buffered formalin, washed with PBS, stained for 30 minutes at 37°C in 0.1 g/mL propidium iodide, 100
g/mL RNAse A, in PBS pH 7.2 and mounted in 90% glycerol solution. The slides were examined with a Zeiss microscope (Carl Zeiss Inc., Thornwood, NJ, USA) using an ultraviolet light source filtered for propidium iodide. Images were photographed on Kodak TMAX 3200 film and printed at equivalent exposures.
Internucleosomal DNA fragmentation of genomic DNA, a characteristic of apoptosis, was assessed both in whole kidney and in MCT cells. For this purpose, kidney samples or 106 cultured cells were lysed in 400 L of hypotonic lysis buffer (100 mmol/L NaCl, 100 mmol/L Tris, 1 mmol/L EDTA, 1% SDS in PBS, pH 7.2) with 200
g/mL proteinase K overnight at 37°C. DNA was precipitated, resuspended and separated in a 1.5% agarose gel. The DNA fragmentation ladder was demonstrated with ethidium bromide staining of the gel and/or Southern blotting. For the Southern blotting, the DNA was transferred onto nylon membranes (Genescreen; NEN, Boston, MA, USA) and probed with MCT genomic DNA which was radiolabeled with [32P]CTP by random priming (Boehringer-Mannheim, Indianapolis, IN, USA)13. Similar results were obtained when the DNA was prepared from 2
106 cells which were lysed in 400
L 0.2% Triton X-100, 10 mmol/L Tris, 1 mmol/L EDTA pH 8. The lysate was then cleared by centrifugation for 15 minutes at 13,800 g. The DNA in the clarified supernatant was precipitated and then processed as described above.
Northern hybridization
For Northern blotting, 30 g of total RNA was separated in 1% agarose gels containing 2.3% formaldehyde29. RNA was transferred to nylon membranes (Genescreen Plus; NEN) and pre-hybridized and hybridized at 65°C in 6
SSC, 5
Denhardts, 10% dextran sulfate, 1% SDS, 100
g/mL salmon sperm DNA and 100
g/mL polyadenylic acid. The probes were labeled by random priming and added to the hybridization solution to a final activity of 1.5
106 cpm/ml. After hybridization, the blots were washed twice in 2
SSPE for 15 minutes at room temperature, once in 2
SSPE, 2% SDS for 45 minutes at 65°C, and once in 0.1
SSPE, 0.1% SDS for 15 minutes at 65°C. The blots were then exposed to film at -70°C with the use of an intensifying screen. Blots were stripped and subsequently rehybridized with the probe for 18S or the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), to account for small loading or transfer variations. For quantitative purposes, the autoradiograph were scanned with a densitometer (Hoefer Scientific Instruments, San Francisco, CA, USA).
Probes for murine Bcl2, murine Bax, murine bclx and human BclxL have already been described29. The murine probes were prepared by PCR of reverse transcribed total RNA isolated from MCT cells. They were cloned and sequenced. The human BclxL probe is specific for the BclxL transcript of the bclx gene, and it has also been cloned and sequenced. The c-myc probe was a gift from Dr. William Lee, Department of Medicine, University of Pennsylvania.
Western blot
Tissue or cell samples were homogenized in lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 2 mmol/L EDTA, 2 mmol/L EGTA, 0.2% Triton X-100, 0.3% NP-40, 0.1 mmol/L PMSF and 1 g/ml pepstatin A) then separated by 12% SDS-PAGE under reducing conditions. After electrophoresis, samples were transferred to PVDF membranes (Millipore Corp., Bedford, MA, USA), blocked with 5% skimmed milk in PBS/0.5% vol/vol Tween 20 for one hour, washed with PBS/Tween, and incubated with the following antibodies: rabbit polyclonal anti-BclxS/L antibodies (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-Bax antibodies (1:2000; Pharmingen, San Diego, CA, USA), rabbit anti-Bcl2 antibodies (1:2000; Pharmingen), and anti-TNF-
antibodies (1:200; Santa Cruz Biotechnology). All antibodies were diluted in 5% milk PBS/Tween. Blots were washed with PBS/Tween and subsequently incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:2000; Amersham, Aylesbury, UK). After washing with PBS/Tween, the blots were developed with the enhanced chemiluminescence method (ECL) following the manufacturer's instructions (Amersham).
Immunohistochemistry
Paraffin-embedded tissue sections, 5 m thick, were floated onto APES (Sigma) coated slides. Slides were deparaffined with xylene and dehydrated in graded concentrations of ethanol. Endogenous peroxidase was quenched with 3% H2O2:methanol (1:1) for 30 minutes at room temperature. Sections were rinsed twice in PBS and then blocked with 6% horse serum and 4% BSA in PBS for one hour at room temperature. Primary antibodies were: rabbit polyclonal anti-BclxS/L (1:100; Santa Cruz Biotechnology), rabbit anti-Bax serum (1:1000; Pharmingen), rabbit anti-Bcl2 serum (1:200; Pharmingen). They were diluted in 1% horse serum and 4% BSA in PBS and left overnight at 4°C. Sections were washed twice for five minutes in PBS, followed by the addition of horseradish peroxidase conjugated anti-rabbit IgG (Amersham) at a dilution 1/200 in 4% BSA in PBS for 30 minutes at room temperature. After washing twice for five minutes in PBS, antibody location was determined with the addition of DAB chromogen (Dako, Glostrup, Denmark):3% H2O2 (130:1) for 10 to 15 minutes. Color development was stopped by washing in water. Sections were counterstained with Carazzi's hematoxylin (Bio-optica, Milano, Italy), dehydrated and mounted in Canada balsam (DPX, Poole, UK). As the negative control, nonspecific rabbit IgG was used in the place of the primary antibody.
Statistics
Results are expressed as mean SEM. Significance at the 95% level was established using one-way ANOVA and Student's t-test. The presence of significant differences between groups was examined by a post hoc test (Bonferroni's method) by means of the SigmaStat statistical software (Jandel, San Rafael, CA, USA).
RESULTS
Acute renal failure induced by folic acid is associated with changes in the expression of cell death genes
Folic acid nephropathy is a classical model of ARF with tubular injury30. Microscopic examination of kidney tissue from mice with ARF revealed the presence of tubular epithelial cells with pyknotic nuclei typical of apoptosis not present in control kidneys Figure 1a. In addition, the genomic DNA from kidneys harvested within 24 hours of folic acid administration showed internucleosomal fragmentation which was not present in control kidneys Figure 1b. Mean serum creatinine was fivefold increased in mice injected with folic acid with respect to vehicle-injected mice (1.5 0.2 vs. 0.3
0.01 mg/dL, P < 0.05).
Figure 1.
Apoptosis in acute renal failure (ARF). (A) Apoptotic cells were noted in tubuli from mice with ARF after 24 hours of folic acid injection. Note apoptotic cells with condensed chromatin in the tubular lumen (H & E, original magnification 1000). No such cells were observed in normal kidneys. (B) Internucleosomal DNA degradation was present at 24 hours in kidneys from mice with ARF, but not in normal kidney (N).
To assess changes in mRNA transcript levels of various cell death genes, Northern blot analysis was performed on RNA isolated from murine kidneys at various times after the administration of folic acid or control vehicle. Six hours after administration of folic acid, there were elevations in mRNA levels encoding c-myc, Bax, and Bclx, while mRNA levels encoding Bcl2 fell Figure 2a. The up-regulation of bclx expression was also observed with the use of a BclxL-specific probe.
Figure 2.
Cell death genes in ARF induced by folic acid. (A) Northern blot. Gels were loaded with 30 g of total RNA isolated from whole kidneys of mice that had been injected with folic acid or vehicle. Membranes were sequentially hybridized with probes for the indicated cell death genes. (B) Cell death gene expression changes during the first 48 hours of evolution of disease. Three different blots from different mice were analyzed by densitometry, and results expressed as arbitrary densitometry units normalized to GAPDH mRNA. Data are mean
SEM, *P < 0.05 vs. control. Symbols are (
) Bcl2; (
) BclxL; (
) Bax. (C) Western blot (24 h). (D) Western blot densitometry in (
) control and (
) ARF mice. Mean of 2 mice.
mRNA encoding c-myc showed a peak 25-fold elevation at six hours, Bax showed a peak 3.6-fold elevation at 24 hours, and BclxL showed a peak threefold elevation at 24 hours Figure 2b. The mRNA encoding Bcl2 decreased by 40% at six hours and remained depressed for 48 hours. The mRNA levels for all of these genes returned toward normal by 72 hours and remained normal at seven days. As peak changes in the renal expression of cell death genes occurred at 24 hours, Bcl2, BclxL and Bax proteins were assessed 24 hours after injection of folic acid. Western blot demonstrated that BclxL, but not BclxS was detectable in control and ARF kidneys Figure 2c. Bcl2 protein decreased 30%, BclxL increased fourfold and Bax increased slightly.
Protein immunohistochemistry revealed that the distribution of increased BclxL protein in ARF was patchy, with some tubules showing intense staining while some cells were negative Figure 3 b, c. By contrast, a diffuse decrement in Bcl2 staining was noted Figure 3e. Vascular smooth muscle cells were the main site of Bax expression in the normal murine kidney Figure 3f. In ARF increased expression of Bax was patchy in whole tubuli and isolated tubular cells, including those detached into the tubular lumen Figure 3g. No significant staining was found in glomeruli with any of the antibodies.
Figure 3.
Immunohistochemistry of kidneys after 24 hours of the injection of folic acid or vehicle. (A–C) BclxL. Tubuli with both increased and decreased staining are present in ARF (D, E) Bcl2. A diffuse decrement in Bcl2 is noted in ARF. (F, G) Bax. Note detached tubular cells stained with anti-Bax in G. (H, I) Negative control stained in which nonspecific IgG was used as primary antibody. (A, D, F, H) Control kidneys; (B, C, E, G, I) ARF kidney, (original magnification 400, except C,
1000).
TNF- has also been shown to play a role in other models of acute renal failure14,15. Interestingly, increased renal TNF-
expression was also noted 24 hours after disease induction in this model of ARF (twofold increase over control; Figure 2c).
Death of cultured tubular cells induced by serum deprivation and TNF-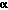 stimulation has features of apoptosis
stimulation has features of apoptosis
A relative deficit of cytokines with survival factor activity has been reported in ARF7,8,9,10, and we tested the effect of depriving cultured tubular epithelial cells of the survival factors present in serum. MCT cells grown in serum-free conditions accumulate more slowly than those grown in media supplemented with 10% FCS (cell number after 72 h in 10% FCS 2190 54 vs. 116
6
102 cells in serum-free media, P < 0.0001; Figure 4a). While decreased cell proliferation in the absence of serum has been previously noted31, increased cell death also contributed to lower cell numbers Figure 4b. After 72 hours in serum-free culture 35.9
2% of the cells were dead, versus 9.6
1% in the controls grown in the presence of 10% FCS (P < 0.0001). Dead cells had characteristic features of apoptotic cell death. Apoptotic bodies were observed among serum-deprived cells Figure 5a. Loss of cell contact is an early feature of apoptosis32. An increased number of detached cells with pyknotic nuclei were also noted in the supernatants of the MCT cells grown in serum-free conditions. While genomic DNA from the attached cells grown in serum-free conditions displayed internucleosomal DNA fragmentation, this pattern was much more evident in the detached cells Figure 5b. To further estimate the cell death attributable to apoptosis, Southern blot analysis was performed on the DNA obtained from detached cells floating in the culture supernatant with probe being total genomic DNA. The radioactivity bound to the low molecular weight DNA obtained from floating cells was counted and then normalized for the number of attached cells. Low molecular weight DNA isolated from supernatants after 72 hours of serum-free culture bound fourfold more counts than DNA isolated from the supernatants of the culture grown in the presence of 10% FCS.
Figure 4.
Serum deprivation and addition of TNF- are cytotoxic for MCT cells. (A) Both serum deprivation and addition of 30 ng/mL TNF-
resulted in MCT cell death, as assessed by trypan blue exclusion. The combination of the two stimuli increased cell death. (B) Increased cell death was associated with decreased cell accumulation. Ten thousand MCT cells were seeded in 24-well plates and grown overnight in 10% FCS DMEM. Then, the medium was changed to either 10% FCS DMEM (
), 10% FCS DMEM with 30 ng/mL TNF-
(+), or serum-free DMEM (*). After 24 hours in serum-free medium, 30 ng/mL TNF-
was added to some wells (
). Mean of quadruplicate wells (SEM < 5%).
Figure 5.
Cell death induced by serum deprivation and TNF- had features of apoptosis, such as the typical morphology, and internucleosomal DNA fragmentation. (A) Apoptotic bodies are observed in propidium iodide stained MCT cells grown in serum-free medium for 48 hours (arrows). By contrast, mitotic figures were prominent in cells grown in 10% FCS DMEM (arrows). (B) Internucleosomal DNA fragmentation of detached cells is already evident after culture for 24 hours in serum-free medium in the presence of 30 ng/mL TNF-
. After 48 hours, fragmented DNA is also present in cells cultured in control serum-free medium, but more prominent in cells cultured in the presence of TNF-
. (C) Flow cytometry of permeabilized, propidium iodide-stained control cells grown in 10% FCS, serum-depleted cells (SF) and serum-depleted cells treated for 48 hours with 30 ng/mL TNF-
. Note the apoptotic cells with hypodiploid DNA content.
In addition to growth factor deprivation, we wanted to examine whether factors known to induce apoptosis in other cells would induce apoptosis in MCT cells. One such factor, TNF-, is increased in ARF induced by folic acid (above). Therefore, TNF-
at a final concentration of 30 ng/mL was added to the culture media of cells growing in the presence of 10% serum or cells that had been serum-deprived for 24 hours. TNF-
increased the rate of cell death in MCT cells more markedly in the serum-free cultures than in the serum containing cultures. Under serum-free growth conditions 80.1
1.2% of the cells exposed to TNF-
were dead at 72 hours, compared to 43.8
5% in the control cultures (P < 0.01) as assessed by trypan blue exclusion Figure 4. Consistent with this increased cell death, the total cell number at 72 hours was also decreased in TNF-
-treated cells (TNF-
900
63, serum-free control 6023
214 cells/well, P < 0.01). The cytotoxic effect of TNF-
was less marked in the presence of serum (TNF-
15.8
0.9%, 10% FCS control 9.5
1% dead cells at 72 h, P < 0.01). The cell death induced by TNF-
had characteristics of apoptosis. The number of cells floating in the supernatant of cultures to which TNF-
was added was threefold greater by 48 hours than the comparable control culture. Examination of these floating cells confirmed apoptotic changes including pyknotic nuclei (not shown) and internucleosomal DNA fragmentation Figure 5b.
Flow cytometry confirmed the presence of apoptotic hypodiploid cells (Ao peak) among those treated with serum deprivation or addition of TNF- Figure 5c, the magnitude of cell death being similar to that obtained in trypan blue exclusion experiments (data not shown).
mRNA levels of apoptosis regulatory genes and their protein products in MCT cells change during serum deprivation and TNF-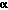 stimulation
stimulation
Serum deprivation of MCT cells modulated the expression of genes that regulate apoptosis. Bax mRNA expression was elevated 24 to 72 hours after withdrawal of serum (1.6 0.15-fold at 48 h, peak 2.9-fold at 72 h), while BclxL and Bcl2 message levels decreased (0.72
0.06 and 0.62
0.14 at 48 h; Figure 6 a, b). These changes result in decreased Bcl2/Bax and BclxL/Bax ratios, which reflect a change in the balance between cell life- and death-promoting genes (Bcl2/Bax and BclxL/Bax mRNA ratios in cells deprived of serum for 48 hours were 39% and 44% those of cells grown in serum). Protein expression was studied by Western blot at 48 hours. Bax protein expression increased twofold, while that of BclxL decreased 45% and Bcl2 decreased 30% Figure 6c. The Bcl2/Bax and BclxL/Bax protein ratios in cells deprived of serum for 48 hours decreased Figure 6d.
Figure 6.
Deprivation of the survival factors present in serum modulates the expression of cell death genes and their products. (A) Northern blot. Gels were loaded with 30 g of total RNA obtained from MCT cells grown in either 10% FCS or in serum-free medium for 48 hr. Membranes were sequentially hybridized with Bcl2, BclxL, Bax and 18S. (B) Bcl2/Bax and BclxL/Bax mRNA ratios after 48 hours of serum deprivation (mean
SEM of 3 independent experiments; *P < 0.05). (C) Western blot. Cells were cultured for 48 hours in the presence of 10% FCS or under serum-free conditions. (D) Western blot densitometry. Mean of 2 independent experiments: Bcl2/Bax and BclxL/Bax protein ratios after 48 hours of serum deprivation in (
) FCS or (
) serum-free conditions.
Addition of TNF- to serum-deprived cells resulted in further changes in the expression of apoptosis genes. The earliest change was a 3.5-fold increase in c-myc mRNA that was already evident at one hour Figure 7a. The mRNA encoding Bcl2 reached a nadir at eight hours (45% of control), with almost total recovery by 48 hours while mRNA encoding BclxL decreased progressively with a nadir at 48 hours (60% of control) Figure 7 a, b. Western blot demonstrated that TNF-
did not induce changes in Bax protein Figure 7 c, d nor did it induce BclxS expression (not shown). TNF-
also did not appreciably change Bcl2 protein levels but it reduced BclxL protein 30% at 48 hours Figure 7 c, d.
Figure 7.
TNF- modulates the expression of cell death genes and their products. (A) Northern blot. Gels were loaded with 30
g of total RNA obtained from MCT cells grown in serum-free medium for 24 hours, before adding either control media or 30 ng/mL TNF-
for 1 to 48 hours. Membranes were sequentially hybridized with Bcl2, Bclx, Bax, c-myc, and 18S. (B) Densitometry of the previous membranes. RNA expression was normalized for 18S, and results are expressed in arbitrary densitometry units in relation to controls cultured for similar time periods (
-Bax;
-Bcl2;
-Bclx). Mean
SEM of three independent experiments. *P < 0.05 versus control. (C) Western blot of MCT cells cultured for 48 hours in the presence or absence of 30 ng/mL TNF-
. (D) Western blot densitometry (
-Control;
-TNF-
). Mean of 2 independent experiments.
BclxL overexpression protects from TNF-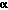 induced apoptosis
induced apoptosis
A reduced BclxL/Bax ratio may contribute to the increased sensitivity of serum-deprived MCT cells to death induced by TNF-. Supporting this hypothesis, overexpression of BclxL reduced the amount of apoptosis induced by TNF-
in serum-deprived cells by 71% when compared with control vector-expressing cells, as assessed by flow cytometry of DNA content Figure 8.
Figure 8.
BclxL protects from TNF--induced apoptosis. Apoptosis induced at 48 hours by 30 ng/mL TNF-
in serum-deprived cells overexpressing BclxL or control cells was quantified by flow cytometry. Mean
SEM of 5 independent experiments.
Top of page
DISCUSSION
Cell death by apoptosis is known to occur in the kidney during ARF1,2, and we have confirmed its presence in ARF induced in mice by folic acid. Cell death results from the interplay and balance of survival and mortal signals5. From this perspective, an absolute or relative deficit in survival factors in damaged kidney facilitates renal cell death. Cell death is also modulated by a changing balance in protective and lethal intracellular proteins19. We now report changes in the expression of apoptosis regulatory proteins in an experimental model of ARF. Some features of this process in damaged kidney are reproducible in cultured tubular epithelial cells. As both decreased expression of cytokines with survival factor activity and the participation of cytokines that can promote cell death, such as TNF-, have been reported in ARF7,8,9,10,14,15, we subjected cultured tubular cells to deprivation of survival factors (serum deprivation) and/or the addition of a lethal factor (TNF-
). This microenvironment resulted in apoptotic cell death of tubular MCT cells and in changes in the expression of apoptosis regulatory genes.
The administration of folic acid induced ARF, increased renal levels of mRNA encoding c-myc and modulated the expression of genes encoding members of the Bcl2-like family of proteins in the kidney. Under similar conditions, levels of mRNA encoding Bcl2 were decreased, while the message of its endogenous antagonist, Bax, increase. This resulted in a decreased Bcl2/Bax ratio that favored cell death18. By contrast, levels of mRNA encoding BclxL were elevated early in injury and were associated with a patchy increase in BclxL protein in tubules. These changes resemble those reported in a well-characterized model of epithelial cell apoptosis where involution of the mammary gland is associated with weaning. Apoptosis of mammary epithelial cells is also associated with an increased expression of c-myc, Bax and Bcl2 and decreased Bcl2 mRNA33,34,35. In vivo expression of Bcl2-related genes has also been studied extensively in the central nervous system. In these studies decreased levels of Bcl2 and increased Bax mRNA is associated with apoptosis induced by ischemia or in amyotrophic lateral sclerosis36,37.
In ARF, however, there have been conflicting reports regarding the expression of mRNA encoding Bcl2. Increased, decreased or unchanged levels were found in ARF in rats given glycerol or HgCl2, made ischemic, or following unilateral ureteral obstruction38,39,40. The reasons for such divergent findings are unclear. They may reflect different degrees or mechanisms of tissue injury or differences between species. In this regard, levels of renal Bcl2 were reported preliminarily to be low during obstructive renal damage39 and obstruction of renal tubuli participates in the pathogenesis of folic acid nephropathy23. In folic acid nephropathy Bcl2 expression decreased globally in tubular cells. However, it is conceivable that in other models of ARF or in other species, there may be a compensatory increase in Bcl2 in certain cells or tubules. Increased levels of mRNA encoding Bax during ARF has also been reported after renal ischemia in rats40. Immunohistochemistry revealed that in folic acid nephropathy increased Bax expression is not widespread among tubular cells, but rather, it is increased in individual tubules and tubular cells, probably those destined to die. There data are consistent with previous findings in the central nervous system and other systems41,42.
In cultured tubular MCT cells we identified a factor that may contribute to decreased expression of intracellular survival proteins and increased Bax expression in ARF. Survival factor deprivation by lowering levels of serum in culture media increased levels of Bax mRNA and protein, and decreased the Bcl2/Bax and BclxL/Bax ratios. This pattern of gene expression favors cell death18,43. Increased Bax expression has been associated with apoptosis induced by withdrawal of survival factors in nonrenal cells, including epithelial cells44. In fact, evidence from neurons harvested from Bax-deficient mice suggests that Bax is required for apoptosis induced by deprivation of survival factors45. Deprivation of the survival factors present in serum also decreased BclxL mRNA and protein in cultured tubular cells. These findings are consistent with the patchy changes observed in vivo in the expression of BclxL and Bax. Indeed, the variability in expression of cytokines with survival activity in tubular cells, like the decrease in IGF-1 (with increased numbers of IGF-1 receptors) observed in folic acid nephropathy7, suggests that tubular epithelium may compete for limiting amounts of survival factors by up-regulating their receptors, and some cells may even become deprived of them5.
We therefore hypothesize that rate-limiting competition for survival factors contributes to the patchy increment in BclxL expression in ARF. Cells that do not have access to survival factors down-regulate BclxL. In contrast, the response of cells to cytokines with survival factor activity is to increase expression of BclxL and be protected from cell death. This notion is consistent with the findings of increased levels of BclxL in surviving neurons within ischemic areas of the brain, while damaged neurons had decreased BclxL42. Increased expression of proteins that protect from apoptosis by surviving tubular cells may promote tubular regeneration during the recovery phase of ARF. Indeed, renal regeneration in folic acid nephropathy may be exuberant and lead to increased kidney weight46. The degree of protection afforded by BclxL depends on the balance of moieties that favor apoptosis. BclxS may counterbalance the protective effect of an excess amount of BclxL expression35. However, expression of BclxS could not be detected in either our in vitro or in vivo systems.
Serum-deprived tubular cells showed a pro-death pattern of cell death protein expression (decreased Bcl2/Bax and BclxL/Bax ratios) and were, indeed, more susceptible to apoptosis induced by TNF-, a cytokine that promotes tubular cell death and is increased in ARF induced by folic acid. In this regard, enforced BclxL overexpression protected from TNF-
-induced apoptosis. In addition, TNF-
progressively lowered the expression of mRNA encoding BclxL. These findings are consistent with previous reports of TNF-
being able to down-regulate the expression of Bclx in nonrenal cells47. The time course of the changes in Bclx expression is consistent with a role in the delayed effect of TNF-
on cell survival. By contrast, TNF-
transiently decreased Bcl2 mRNA while Bcl2 protein did not change. This dissociation between Bcl2 mRNA and protein expression has been described in other cell systems, and may be related to the long half-life of the Bcl2 protein48,49 or to differential regulation of mRNA and protein half-lives50,51. The transient nature of the decrement in levels of mRNA encoding Bcl2 may prevent Bcl2 protein from falling in MCT cells.
The Bcl2 family of proteins includes other members that protect from (Bclw, A1) or promote (Bad, Bak, BH3-only proteins) cell death19. At least some of them are expressed by tubular epithelium and could play a role in ARF. In addition, Bcl2 and BclxL can prevent cell death with features of necrosis20, and it is conceivable that their role during ARF extends beyond apoptosis to influence other forms of cell death.
Increased c-myc mRNA had been previously reported in the early stages of folic acid nephropathy and other models of ARF52,53, where it was thought to play a role in compensatory cell proliferation. However, recent data suggest that cell proliferation and apoptosis may share a common initial pathway, and that availability of additional external survival factors and the expression of cell death protection genes may better determine cell fate. Overexpression of c-myc leads to cell proliferation in the presence of serum or high levels of intracellular protective proteins, but cells with deregulated c-myc expression undergo apoptosis if survival factors are withdrawn or Bcl2 levels are low17,54. In a similar manner, we propose that increased levels of c-myc in ARF may regulate cell number depending on the local availability of survival factors. The increased sensitivity to TNF--induced cell death of serum-deprived tubular cells may be related to the high c-myc expression induced by TNF-
. Induction of c-myc expression had previously been associated with increased TNF-
cytotoxicity55 and survival factors also prevent TNF-
-induced cell death in oligodendrocyte cultures4.
Taken together, the in vitro data demonstrating an influence of extracellular factors with survival and lethal activity on the expression of apoptosis regulatory proteins in tubular cells, the in vivo data of similar changes in ARF, and previous evidence for absolute or relative deficit of cytokines with survival factor activity and increased release of lethal factors during ARF, suggest that apoptosis regulatory proteins like c-Myc, Bcl2, Bax and Bclx may have a role in the regulation of tubular cell death during ARF. The precise role of each of these proteins in renal disease remains to be clarified. The better understanding of the role and regulation of apoptotic cell death in acute renal injury may provide new insights into the pathogenesis of ARF and provide the basis for new therapeutic strategies. Understanding the regulation of tubular cell apoptosis may be also relevant to the pathogenesis of tubular atrophy in chronic renal disease3. Tubular cell apoptosis has been observed in this setting and lethal cytokines, such as TNF- and Fas ligand, may leak from the inflamed glomeruli and reach the tubular epithelium or may be released from infiltrating lymphocytes or interstitial fibroblasts.
References
References
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. Top of pageAcknowledgments
Alberto Ortiz was supported by grants MEC 95/0093, SAF 97/0071, FIS 98/0637 and Instituto Reina Sofia de Investigaciones Nefrologicas. Corinal Lorz was supported by Ministerio de Educación y Ciencia, and Marina Catalán by Fundación Conchita Rabago. This work was also supported in part by grants DK-46282, DK-07006, DK-30280, and DK-55926 from the National Institutes of Health.
Main navigation
ISN resources
- Nephrology Gateway
- Chinese edition
- Spanish edition
- Portuguese edition
- Contact us
- Society news
NPG resources
- KI Japanese home page
- Nature Reviews Nephrology
- Nature Reviews Urology
NPG Journals
by Subject Area
-
Chemistry
- Chemistry
- Drug discovery
- Biotechnology
- Materials
- Methods & Protocols
Clinical Practice & Research
- Cancer
- Cardiovascular medicine
- Dentistry
- Endocrinology
- Gastroenterology & Hepatology
- Methods & Protocols
- Pathology & Pathobiology
- Urology
Earth & Environment
- Earth sciences
- Evolution & Ecology
Life sciences
- Biotechnology
- Cancer
- Development
- Drug discovery
- Evolution & Ecology
- Genetics
- Immunology
- Medical research
- Methods & Protocols
- Microbiology
- Molecular cell biology
- Neuroscience
- Pharmacology
- Systems biology
Physical sciences
- Physics
- Materials
by A - Z Index
Extra navigation
.ARTICLE NAVIGATION - FULL TEXT
Previous | Next- Table of contents
- Download PDF
- Send to a friend
- Order Commercial Reprints
- CrossRef lists 28 articles citing this article
- Scopus lists 71 articles citing this article
- Save this link
- Abstract
- METHODS
- RESULTS
- DISCUSSION
- References
- Acknowledgments
- Figures and Tables
- Export citation
- Export references
- Papers by Ortiz
- partner of AGORA, HINARI, OARE, INASP, CrossRef and COUNTER