Kidney International - Overexpression of chem...
来源:百度文库 编辑:神马文学网 时间:2024/05/01 09:16:05
- Jump to main content
- Jump to navigation
- nature.com homepage
- Publications A-Z index
- Browse by subject
- ISN
- My account
- Submit manuscript
- Register
- Subscribe
Login
Cell Biology – Immunology – Pathology
Kidney International (2000) 57, 147–158; doi:10.1046/j.1523-1755.2000.00830.x
Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy
Sergio A Mezzano, M Alejandra Droguett, M Eugenia Burgos, Leopoldo G Ardiles, Claudio A Aros, Italo Caorsi and Jesús Egido
Division of Nephrology, School of Medicine, Universidad Austral, Valdivia, Chile, and Fundación Jiménez Díaz, Universidad Autónoma, Madrid, Spain
Correspondence: Sergio Mezzano, M.D., Department of Nephrology, School of Medicine, Universidad Austral, P.O. Box 8-D, Valdivia, Chile. E-mail: smezzano@uach.cl
Received 18 February 1999; Revised 12 August 1999; Accepted 23 August 1999.
Top of pageAbstract
Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy.
Background
Proteinuria plays a central role in the progression of glomerular disease, and there is growing evidence suggesting that it may determine tubular cell activation with release of chemokines and fibrogenic factors, leading to interstitial inflammatory reaction. However, most studies on this subject have been performed in experimental models, and the experience in human kidney biopsies has been scarce. We analyzed the tissue sections of patients with idiopathic membranous nephropathy (IMN), a noninflammatory glomerular disease that may follow a progressive disease with heavy persistent proteinuria, interstitial cell infiltration, and decline of renal function.
Methods
Paraffin-embedded biopsy specimens from 25 patients with IMN (13 progressive and 12 nonprogressive) were retrospectively studied by immunohistochemistry [monocyte chemoattractant protein-1 (MCP-1), regulated on activation normal T-cell expressed and secreted chemokine (RANTES), osteopontin (OPN), platelet-derived growth factor-BB (PD-GF-BB)] and in situ hybridization [MCP-1, RANTES, PDGF-BB, transforming growth factor-1 (TGF-
1)]. Moreover, we studied the presence of myofibroblasts, which were identified by the expression of
-smooth muscle actin (
-SMA), the monocytes/macrophages (CD68-positive cells), and T-cell infiltration (CD4+ and CD8+ cells). All of the patients were nephrotic and without treatment at time of the biopsy.
Results
A strong up-regulation of MCP-1, RANTES, and OPN expression was observed, mainly in tubular epithelial cells, with a significant major intensity in the progressive IMN patients. A strong correlation between the mRNA expression and the corresponding protein was noted. The presence of these chemokines and OPN was associated with interstitial cell infiltration. TGF- and PDGF were also up-regulated, mainly in tubular epithelial cells, with a stronger expression in the progressive IMN, and an association with the presence of myofibroblasts was found.
Conclusions
Patients with severe proteinuria and progressive IMN have an overexpression in tubular epithelial cells of the chemokines MCP-1, RANTES, and OPN and the profibrogenic cytokines PDGF-BB and TGF-. Because this up-regulation was associated with an interstitial accumulation of mononuclear cells and an increase in myofibroblastic activity, it is suggested that those mediators are potential predictors of progression in IMN. Finally, based on experimental data and the findings of this article, we speculate that severe proteinuria is the main factor responsible for the up-regulation of these factors in tubular epithelial cells.
Keywords:
monocyte chemoattractant protein-1; osteopontin, growth factors, proteinuria, idiopathic membranous nephropathy
In glomerular diseases, severe proteinuria usually is associated with faster progression, and this correlation is even stronger for patients with nephrotic range proteinuria1,2,3. A highly significant correlation between baseline urinary protein excretion and subsequent glomerular filtration rate decline has been shown4. Moreover, a number of studies in the last 10 years have indicated that in human glomerular diseases, the severity of proteinuria correlated with tubulointerstitial infiltrates and that the degree of tubulointerstitial injury predicts renal function decline better than the severity of glomerular damage5,6,7,8,9.
However, the mechanisms underlying the involvement of the tubulointerstitium in progressive glomerulonephritis remains largely unknown.
There is a growing evidence that abnormal glomerular permeability to proteins causes proximal tubular cell dysfunction1 and that proteinuria elicits tubular activation, with overexpression of chemokines10,11,12 and fibrogenic cytokines10,13.
In rats with protein-overload proteinuria, a model with interstitial inflammation14, renal monocyte chemoattractant protein-1 (MCP-1) mRNA and osteopontin (OPN) mRNA levels were up-regulated, and MCP-1 protein was mainly localized in the tubules10, suggesting that both molecules could be implicated in interstitial inflammation. More recently, in the experimental model of Heymann nephritis, OPN was detectable in proximal tubule cells congested with protein at the sites of interstitial inflammation12. In addition to MCP-1 and OPN, a third powerful chemoattractant produced by activated tubular cells has been proposed: regulated on activation normal T-cell expressed and secreted chemokine (RANTES)15.
Besides the tubular cell activation and immune cell infiltration, the presence of interstitial myofibroblasts -smooth muscle actin-positive (
-SMA+) cells, which can be modulated by transforming growth factor-
(TGF-
)16,17 and platelet-derived growth factor (PDGF)18, has been proposed to be the main component in the pathogenesis of tubulointerstitial injury19.
In this study, we simultaneously analyzed the expression of MCP-1, RANTES, OPN, and profibrogenic cytokines PDGF-BB and TGF- by immunohistochemistry (IMH) and in situ hybridization (ISH) in renal biopsy sections of patients with human idiopathic membranous nephropathy (IMN), as it is a noninflammatory glomerular disease characterized mainly by the presence of nephrotic proteinuria. As IMN may manifest a progressive decline in renal function20 and show persistently high levels of urinary protein excretion and tubuloinsterstitial lesions at biopsy, we also focused on the relationship between chemokines and growth factor expression in the presence of interstitial cell infiltration, particularly interstitial myofibroblasts (
-SMA positive cells), monocyte/macrophages (CD68-positive cells), and T lymphocytes (CD4+ and CD8+) in the tissue sections.
METHODS
Human kidney specimens
Kidney samples were obtained by percutaneous renal biopsy from patients undergoing diagnostic evaluation at the Division of Nephrology, Austral University, Valdivia, Chile. Control human kidney specimens (N = 5) were taken from normal portions of nephrectomy from patients who underwent surgery because of localized renal tumors. Renal biopsies from 25 patients with idiopathic membranous glomerulonephropathy (MGN; 13 with progressive IMN and 12 with nonprogressive IMN) were studied. The diagnosis of IMN was made based on clinical and histologic findings, and tests were done to exclude secondary causes of membranous nephropathy. All of the patients were screened for the presence of antinuclear antibodies, anti-DNA antibodies, rheumatoid factor, and hepatitis B antibodies. C3 and C4 were in the normal range in all of the cases, and parasitic infections were discarded. Blood levels of carcinoembryonic antigen were determined when appropriate. Patients who were exposed to any drug associated with this disease were also excluded.
The classification of progressive IMN, based on the presence of a severe nephrotic syndrome and a reduction of renal function at the time of the biopsy (cases 2,4, 5, 6, and 9), was done in 9 out of 13 patients, and was confirmed in these patients because their disease progressed to chronic renal failure during an average of 4.3 years (range 2 to 10) of clinical follow-up. The remaining four patients (cases 10 to 13) were biopsed and classified as progressive during the last two years of the study, based on the presence of a severe nephrotic syndrome, a decrease in renal function, and interstitial cell infiltration. Case 10 had already progressed to chronic renal failure during this time.
The nonprogressive IMN patients were nephrotic and with a stable renal function at the time of the biopsy. They had no decline in renal function during the long-term follow-up, which was between 3 and 25 years (mean follow-up 11 years).
All of the patients were without treatment at the time of the biopsy, and no patient had renal vein thrombosis.
The histologic diagnosis and determination of the stage of idiopathic MGN was done by light, fluorescence, and electron microscopy. For light microscopy, kidney tissues were fixed in 4% buffered formalin or Bouin overnight, and were dehydrated and embedded in paraffin by conventional techniques. Sections were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and silver methenamine; some sections were stained with Van Gieson in order to determine the presence of interstitial collagen.
Immunohistochemistry
Paraffin-embedded biopsy specimens were used for detection of MCP-1, RANTES, OPN, PDGF-BB, -SMA, and CD68 (a marker of macrophages). Specific biotinylated secondary antibodies that were followed with streptoavidin-horseradish peroxidase conjugate (Dako, Carpinteria, CA, USA) and revealed with DAB were used.
The following primary antibodies were employed: MCP-1 (polyclonal goat antihuman, AB-279-NA; R&D Systems, Minneapolis, MN, USA); RANTES (polyclonal goat antihuman, AB-278-NA; R&D Systems); OPN (mouse monoclonal, MPIIIB10; DSHB, USA); PDGF-BB (polyclonal rabbit antihuman, ZP-215; Genzyme, Cambridge, MA, USA), -SMA (mouse monoclonal
-SMA, 1A4; Dako), and CD68 (mouse monoclonal PG-M1; Dako). Briefly, 5-micron thick renal sections, Bouin or formalin fixed and paraffin embedded, were adhered to polylysine-covered glass and fixed overnight at 56°C. After deparaffinizing through xylene, alcohol, and distilled water, endogenous peroxidase was blocked by 0.3% H2O2 for 20 minutes. The sections were then treated in a microwave oven in a solution of citrate buffer, pH 6.0, for 20 minutes and transferred to distilled water. After rinsing in Tris-HCl phosphate-buffered saline (TPS), the sections were incubated with 1:10 normal rabbit serum in TPS/1% bovine serum albumin (BSA). Dako protein block serum-free (cat. X0909) was used with the anti–PDGF-BB antibody for 30 minutes at 22°C. The sections were then incubated overnight at 4°C with unlabeled polyclonal and monoclonal specific primary antibodies. For the OPN,
-SMA, and CD68 staining, after three rinses in TPS, the sections were incubated with biotinylated rabbit antimouse in a 1:200 dilution in TPS-BSA 1%. For detection of RANTES and MCP-1, the sections were incubated with biotinylated rabbit antigoat, dilution 1:300 in TPS-BSA 1%, and for the detection of PDGF-BB, the sections were incubated with biotinylated swine antirabbit dilution 1:100 in TPS-BSA 1% for 30 minutes at 22°C. Following three rinses in TPS, they were then incubated with streptavidin-peroxidase (Dako) 1:500 for 30 minutes. Color was developed with diaminobenzidine, counterstained with hematoxylin, and then dehydrated and mounted with Canadian balsam (Polysciences, Inc., Warrington, PA, USA). The specificity was checked by omission of primary antibodies and use of nonimmune sera.
Cryostat sections (5 m thick) from tissue fragments, frozen and kept in liquid nitrogen, were also used for the detection of the macrophage marker CD68 (mouse monoclonal PG-M1; Dako) and for T lymphocytes CD4 (monoclonal mouse antihuman T cell, CD4, DAKO-CD4, MT310) and T cells CD8 (monoclonal mouse antihuman T cell, CD8, DAKO-CD8, DK25). Specific biotinylated secondary antibodies, followed by streptoavidin-horseradish peroxidase conjugate and revealed with DAB or AEC, were used. (Dako LSAB2 systems).
In situ hybridization
Paraffin-embedded tissue sections, using different biotin-labeled antisense probe cocktail specific for MCP-1, RANTES, PDGF, TGF-, followed with avidin-alkaline phosphatase conjugate and BCIP/NBT (R&D Systems), were used. The probes of biotin-labeled human MCP-1, RANTES, PDGF, and TGF-
cocktail were purchased from R&D Systems (Minneapolis, MN, USA).
Tissue preparation
Tissue was fixed in 4% buffered formalin, embedded in paraffin wax, and cut into 5 m sections, which were mounted onto 3-aminopropyltriethoxy-silane–coated slides treated with diethylpyrocarbonate (DEPC; Sigma Chemical Co., St. Louis, MO, USA).
Tissue sections were dewaxed with xylene and rehydrated through a series of decreasing ethanol solutions. Slide-mounted sections were treated with 2 standard saline citrate (SSC; 1
0.15 mol/L NaCl in 0.015 mol/L trisodium citrate, pH 7.0) at 60°C for 10 minutes and were washed with DEPC-treated water.
Then tissue sections were digested with proteinase K (5 g/mL) in 0.05 mol/L Tris (pH 7.6) for 60 minutes at 37°C. After a wash with DEPC-treated water, the sections were incubated with a prehybridization solution containing 0.6 mol/L salt, 30% formamide, and 150
g/mL sonicated salmon sperm DNA for 60 minutes at 37°C (R&D Systems).
The hybridization reaction was performed overnight at 37°C with 100 L of biotin-labeled probe cocktails for each cytokine (200 ng/mL; R&D Systems) in a humidified chamber.
Then the slides were washed twice with 4 SSC 30% formamide, twice with 2
SSC 30% formamide, and twice with 0.2
SSC 30% formamide at 37°C for five minutes, and then with 1
Tris-buffered saline (TBS) containing 2% Triton at room temperature for 15 minutes.
Detection was performed with avidin-alkaline phosphatase conjugate (Dako) for 30 minutes at room temperature. It was washed for five minutes with 1 TBS and using nitrobluetetrazolium (NBT) and 5-bromo-4-cloro-3-indolyl phosphate (BCIP) as the enzyme substrate for 120 minutes at 37°C in the dark (R&D Systems). Tissues were then dehydrated in ethanol series and mounted in Canadian balsam.
The specificity of the reaction was confirmed by: (a) demonstrating the disappearance of hybridization signal when RNAse (100 g/mL; Sigma) was added in 0.05 mol/L Tris after the digestion with proteinase K; (b) the use of a sense probe (R&D Systems); (c) a negative control (Plasmid DNA; Dako); and (d) without probe.
Immunohistochemistry coupled to in situ hybridization
This technique was performed to simultaneously detect -SMA–positive cells by IMH and cells expressing TGF-
by ISH on the same biopsy section, as well as to simultaneously detect CD68-positive cells by IMH and MCP-1 by ISH. Renal sections of 5 micron thickness fixed in 4% buffered formalin and embedded in paraffin were sequentially treated for ISH and IMH as reported previously in this article.
Immunohistological examination
Semiquantitative analysis was performed for the IMH and ISH by three different observers in a blinded fashion.
The staining distribution was scored separately in the glomeruli and within the tubulointerstitium. The sections were scored semiquantitatively for the proportion of positive cells according to the following 0 to 3 scale: 0 = no staining; 1 = occasional staining; 2 = focal staining; and 3 = diffuse staining.
As tissue sections were run simultaneously for each assay with the chemokines and growth factors studied (protein and mRNA), it permitted an evaluation of the staining intensity. The intensity of tubulointerstitial and glomeruli staining in cortical areas was evaluated according to the following three-point scale observed at 250 magnification: + = weak; ++ = moderate; +++ = strong intensity of staining.
The mean value was obtained in both scoring systems by dividing the total number of scores by the number of biopsies.
Tubulointerstitial cell infiltration and interstitial fibrosis injury was classified into four groups according to the extent of interstitial cell infiltration and cortical interstitial fibrosis and of tubular atrophy and degeneration: (a) normal, (b) involvement up to 25% of the cortex, (c) involvement of 26 to 50% of cortex, and (d) extensive damage involving more than 50% of the cortex.
Interstitial fibrosis was defined by the presence of interstitial collagen in sections stained with Van Gieson. Staining for -SMA was particularly prominent in areas of fibrosis around atrophic tubules.
The number of CD68-positive monocytes/macrophages and T lymphocytes CD4+ and CD8+ in the interstitium was counted using an ocular grid at a magnification of 400.
Statistical analysis
The results of the clinical data are expressed as the mean SD. The comparison between both groups was performed by the Mann-Whitney U-test. Fisher's test was used to analyze the risk factors of progression in each group.
The score for the intensity and distribution of staining for the IMH and the in situ hybridization in progressive and nonprogressive IMN is expressed as the mean SEM. The comparison between both groups was done by Mann-Whitney U-test. A P value < 0.05 was considered significant.
RESULTS
Clinical and histologic data
Table 1 summarizes the clinical and histologic data for each patient studied, and Table 2 illustrates the comparison between both groups of patients. There was not a significant difference between age and sex. However, the progressive IMN was significantly associated with a reduced renal function (serum creatinine> 1.4 mg/dL) at the time of the biopsy (P = 0.0003). The mean arterial pressure (MAP) over 110 mm Hg (P = 0.0007), a persistent proteinuria 8 g/day for six months or longer (P = 0.0007), advanced glomerular stages on biopsy (P = 0.003), tubulointerstitial cell infiltration (P = 0.01), and with the interstitial fibrosis (P = 0.002) confirmed the prognostic value of these risk factors in patients with IMN21.
Table 1 - Clinical data for patients with idiopathic membranous nephropathy (IMN).
Table 2 - Clinical and histological data.
Immunohistochemistry and in situ hybridization in normal control kidneys
Normal kidneys showed no glomerular staining, and only sparse tubular and interstitial staining of the chemokines (MCP-1, RANTES, and OPN) was found. A very faint staining for PDGF was observed in these kidneys, particularly in the mesangium and the interstitium.
Expression of -SMA was essentially confined to the media of arteries and arterioles. No staining was detected within the glomeruli, and a few sparse
-SMA–positive cells were detected in the peritubular interstitium. Very few macrophages were detected by the CD68 macrophage marker.
In situ hybridization using riboprobes is a sensitive method to study specific mRNA within tissue sections, as it allows a spatial analysis of expression. In the sections of normal kidneys, no specific signal was detected for MCP-1, RANTES, PDGF, and TGF-.
All sections hybridizated with a sense probe were negative, and the binding of the antisense probes to these cells was prevented by preincubation with RNAse.
Detection of proinflammatory chemokines (MCP-1, RANTES, OPN) in idiopathic membranous nephropathy by immunohistochemistry and in situ hybridization
We observed a significant up-regulation of chemokines in almost all of the patients, mainly in tubular epithelial cells Figure 1, with a marked correlation between the MCP-1 mRNA and RANTES mRNA expression. On the other hand, the expression of these proinflammatory cytokines was significantly more intense in the biopsies of patients with the progressive disease, with 10 out of 13 patients having presented a severe nephrotic proteinuria lasting more than six months Figure 4.
Figure 1.
Demonstration of monocyte chemoattractant protein-1 (MCP-1) mRNA by in situ hybridization (ISH), RANTES protein, and osteopontin (OPN) protein by immunohistochemistry in idiopathic membranous nephritis (IMN; magnification 300). (a) MCP-1 mRNA is strongly up-regulated and mainly expressed by tubular epithelial cells in progressive IMN (case 12). (b) MCP-1 mRNA is weakly expressed in tubular epithelial cells in a nonprogressive IMN (case 25). (c) RANTES protein staining is strongly increased in proximal tubular epithelial cells in progressive IMN (case 10). (d) On the other hand, RANTES is weakly increased in tubular cells in a nonprogressive case of IMN (case 24). (e) Strong overexpression of osteopontin protein in tubular epithelial cells in progressive IMN (case 13). Note the interstitial cell infiltration in close relationship with the tubular osteopontin expression. (f) Negative staining for osteopontin in tubular epithelial cells in case of nonprogressive IMN16.
Figure 4.
Schematic summary of chemokines, profibrogenic cytokines, and -SMA expression in progressive (
) and nonprogressive IMN (
). The results of the IMH or ISH staining scores are presented as the mean
SEM. *P < 0.05.
We therefore wondered whether or not the increased mRNA levels for MCP-1 and RANTES corresponded to an increased protein synthesis. Indeed, as evaluated by IMH using a specific polyclonal antibodies, MCP-1 and RANTES protein expression, almost absent in normal renal tissue, was present in 90% of the nephrotic patients with membranous nephropathy, with stronger immunostaining found in those with a progressive disease and with a pattern similar to that observed for mRNA, mainly in tubular epithelial cells. Moreover, by using serial consecutive biopsy sections, we observed the gene and protein expression in the same tubular cells Figures 3 a, b. In glomeruli, there was no mRNA up-regulation of these proinflammatory cytokines, but in some cases, a mild glomerular inmunostaining for MCP-1, RANTES, and OPN was observed.
Figure 3.
(a) Demonstration of MCP-1 mRNA and (b) protein in progressive IMN (case 11; magnification 300). Serial sections of the same biopsy show that MCP-1 mRNA (a) and protein (b) are strongly expressed in tubular epithelial cells. The distribution of MCP-1 mRNA and protein is similar. (c) Immunohistochemistry coupled with ISH for
-SMA positive cells and TGF-
. A moderate increase in interstitial
-SMA is associated with TGF-
mRNA up-regulation in close tubular epithelial cells. (d) CD68-positive cells in progressive IMN (case 10). Paraffin section (magnification
300). (e) CD4+ T-cell lymphocytes in renal interstitium (case 13). Cryostat section (magnification
300). (f) CD8+ T-cell lymphocytes in interstitium (case 13). Cryostat section (magnification
300).
The expression of OPN was studied by IMH using only a monoclonal antibody. There was a clear immunostaining for OPN in approximately 90% of the sections studied that was mainly found in the tubular epithelial cells, similar to MCP-1 and RANTES, and with a stronger expression in the patients with progressive IMN Figure 4.
The increased gene and protein expression of these proinflammatory cytokines (MCP-1, RANTES, and OPN) in the tubular epithelial cells was associated with interstitial cell infiltration and with tubulointerstitial damage Figure 1e. Furthermore, by using serial consecutive biopsy sections, we observed the presence of some CD68-positive cells and mainly -SMA–positive cells (myofibroblats) around those structures in which cells expressed MCP-1, RANTES, and OPN Figure 2e.
Figure 2.
Demonstration of platelet-derived growth factor (PDGF), transforming growth factor- (TGF-
) by ISH, and
-smooth muscle actin (
-SMA) by immunohistochemistry in IMN (magnification
300). (a) Strong up-regulation of PDGF mRNA in tubular epithelial cells in progressive IMN (case 5). (b) Weak up-regulation of PDGF mRNA in tubular epithelial cells in non progressive IMN (case 16). (c) Strong up-regulation of TGF-
mRNA in tubular epithelial cells in progressive case of IMN (case 2). (d) TGF-
mRNA is not up-regulated in nonprogressive IMN case (case 23). (e) Moderate increase in interstitial myofibroblats (
-SMA positive cells in renal interstitium) in a progressive IMN case (case 4). (f) Weak peritubular staining for
-SMA in a case of nonprogressive IMN (case 23).
Again, the sections hybridizated with a sense probe were negative, and binding of the antisense probes to these cells was prevented by preincubation with RNAse.
As the progressive group not only differed in the magnitude of proteinuria, but also had significantly higher blood pressures, more severe histology, and reduction in their renal function, we also compared the staining patterns in those progressive patients who still did not present a significant impairment of renal function (serum creatinine 1.5 mg/dL; cases 1, 3, 4, 6, 7, and 8) versus the nonprogressive patients. The expression of these proinflammatory cytokines remained significantly higher in patients with progressive IMN.
Expression of the profibrogenic cytokines PDGF-BB and TGF-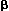
Transforming growth factor- expression was studied only by ISH. TGF-
was up-regulated mainly in cortical tubular epithelial cells, and this expression was significantly higher in the group with progressive disease (100 vs. 40%, P = 0.002; Figure 2 c, d). Furthermore, this group presented a significantly stronger TGF-
expression Figure 4. In general, the TGF-
mRNA expression was increased in the tubulointerstitial area in a manner consistent with the severity of the disease, and particularly, the proximal and distal tubules surrounded by interstitial mononuclear cells were positive Figure 3c. At the glomerular level, we found a weak expression of this growth factor in some patients.
Platelet-derived growth factor mRNA expression was consistent with the expression of TGF- and was observed mainly in cortical tubular epithelial cells and primarily in the cases with progressive disease (9 out of 11 vs. 3 out of 9, P = 0.03; Figure 2 a, b). Furthermore, PDGF mRNA up-regulation was significantly stronger in progressive IMN Figure 4.
Immunostaining for PDGF-BB protein was correlated with the mRNA expression, and it was mainly expressed in tubular epithelial cells, with a staining score significantly higher in those with progressive disease.
When we compared the staining patterns in those progressive patients who were without a significant impairment of renal function (cases 1, 3, 4, 6, 7, and 8) versus the nonprogressive patients, the expression of the profibrogenic cytokines was already significantly higher in patients with progressive IMN.
Again, because all of these IMH and ISH studies for each chemokine and fibrogenic factor were separately but simultaneously performed in all of the tissue sections studied, it allowed us to score them semiquantitatively and to compare the staining intensities.
Interstitial myofibroblast and macrophage infiltration in idiopathic membranous nephropathy
To characterize the proliferating cells in the tubulointerstitial area, tissue sections were immunostained with monoclonal antibodies to identify monocyte/macrophage (cells positive for CD68) or myofibroblasts (cells positive for -SMA).
-Smooth muscle actin interstitial-positive cells were mainly detected in patients with a progressive disease (13 out of 13 progressive vs. 4 out of 12 with nonprogressive, P = 0.0004; Figure 2 e, f). The staining distribution was mainly in the peritubular and periglomerular cortical interstitium, and there was a stronger expression in the patients with a progressive IMN and a weak staining in some positive nonprogressive cases Figure 4. The presence of myofibroblasts was significantly associated with the tubular interstitial cell infiltration (P = 0.02), interstitial fibrosis (P = 0.005), and the severity of the proteinuria (P = 0.03).
In four progressive and three nonprogressive cases, -SMA–positive cells were observed at the glomerular level, which represents a phenotypic modulation of activated mesangial cell. The cell expression was not associated with a higher incidence of glomerulosclerosis.
In a double IMH to -SMA coupled with ISH for TGF-
, performed in some progressive cases, the immunostaining for
-SMA was observed mainly in the peritubular interstitium and TGF-
mRNA expression localized in the surrounding tubular epithelial cells Figure 3c.
Although the tubular interstitial cell infiltration was found in almost all of the progressive IMN biopsy sections, not many cells were recognized as macrophages/monocytes, according to the immunostaining for CD68 Figure 3d. For these reasons and to characterize the mononuclear cell populations infiltrating the interstitium, we looked for the presence of other immune cells, such as CD4+ and CD8+ lymphocytes. Increased numbers of cells with the CD4 and CD8 phenotype were found in the renal interstitium of sections of the progressive patients, and as can be seen in Figure 3 e and f, these were mainly CD4+ cells.
Top of pageDISCUSSION
Human progressive IMN is characterized by a decrease in renal function, persistent nephrotic proteinuria and interstitial cell infiltration20,21. Growing evidence suggests that infiltrating immune cells could be recruited within interstitial areas by three powerful chemoattractants produced by activated tubular cells such as MCP-1, RANTES, and OPN1,15,22,23. MCP-1 and RANTES belong to the family of C-C chemokines [reviewed in 24], and OPN is a secreted acidic glycoprotein that has potent monocyte chemoattractant and adhesive properties25.
Our results document that the C-C chemokines, MCP-1, RANTES, and OPN, are involved in the evolution of progressive IMN. A strong up-regulation of MCP-1, RANTES (mRNA and protein) and OPN protein was demonstrated, mainly in the cortical tubular epithelial cells in renal tissue sections of patients with a progressive disease. On the other hand, the expression of MCP-1, RANTES, and OPN was significantly milder in patients with a nonprogressive disease Figure 4.
The strong up-regulation of these chemokines, mainly localized in the tubules, is consistent with the up-regulation of MCP-1 and OPN observed in the rat with protein-overload proteinuria10 and with Heymann nephritis, an experimental form of membranous nephropathy12,23, and suggests that in human IMN the tubular protein overload up-regulates these inflammatory genes.
In situ analysis of C-C chemokine expression in human glomerulonephritis is mainly limited to MCP-126. Recently, Gesualdo et al demonstrated the expression of MCP-1 mRNA and protein in cryoglobulinemic membranoproliferative glomerulonephritis localized to macrophages and other infiltrating and resident cells27. In renal biopsies from a heterogeneous group of patients with glomerulonephritis, Prodjosudjadi et al analyzed MCP-1 protein expression at glomerular and interstitial sites, but found no correlation between glomerular MCP-1 expression and intraglomerular macrophage infiltration28. In membranous nephropathy, an association was found between the intensity of MCP-1 staining in tubular epithelial cells and interstitial infiltration of macrophages. Grandaliano et al showed an increased MCP-1 gene and protein expression in IgA nephropathy, mainly in cortical tubular epithelial cells, infiltrating mononuclear cells, as well as glomerular parietal cells, which correlated with the presence and the extent of monocyte infiltration and with the severity of the tubulointerstitial lesions22. In biopsies of systemic lupus erythematosus (SLE) patients, Wada et al detected MCP-1 gene and protein in infiltrating monocytes and in cortical tubular cells, but surprisingly not in glomeruli29. Finally, Cockwell et al examined the C-C chemokine expression in a range of human inflammatory glomerulonephritis, demonstrating the concurrent expression of MCP-1, MIP-1, and RANTES mRNAs by glomerular and tubular epithelial cells and colocalizing much of this expression to infiltrating macrophages30.
On the whole, in different human glomerular diseases, a tubular expression of MCP-1 has been observed that is correlated with the monocyte/macrophage infiltration, mainly in the interstitium.
Evidence about the potential role of OPN in glomerular diseases is mainly derived from observations in experimental models of mesangial proliferative nephritis (anti-Thy1 nephritis), focal segmental sclerosis (aminonucleoside nephrosis), and membranous nephropathy (passive Heymann nephritis). These data suggest that: (a) OPN is up-regulated in tubules in glomerular disease; (b) OPN may be important for macrophage accumulation at specific sites during tissue damage; and (c) OPN may have a role in the pathogenesis of the tubulointerstitial injury that accompanies glomerulonephritis12,23.
Indeed, in our study, the increased tubular expression of C-C chemokines, OPN, and fibrogenic growth factors, mainly in the heavily proteinuric progressive IMN patients, clearly suggests an active and central role for tubular epithelial cells in the pathological events involving the renal interstitium. Our data support the hypothesis of Remuzzi, Ruggenenti, and Begnini1, who have suggested that the increased intracellular trafficking of proteins in the tubules could activate the transcription factor nuclear factor-B and consequently increase the expression of a variety of proinflammatory genes (MCP-1, RANTES, and OPN), inducing the recruitment of inflammatory cells.
Although we found a significant interstitial cell infiltration mainly in progressive IMN, our data also suggest that C-C chemokines and OPN might recruit immune cells other than macrophages/monocytes, such as CD4+ and CD8+ T lymphocytes. CD4+ and CD8+ lymphocytes and macrophages were found to accumulate into the renal interstitium in animals with protein-overflow proteinuria14 and more recently in another nonimmune model of proteinuric rats with renal mass reduction31.
Our current data also show a strong increase of tubuloepithelial cells expressing PDGF-BB (mRNA and protein) and TGF- mRNA, which significantly correlates with the degree of tubulointerstitial damage and with the presence of interstitial myofibroblasts (
-SMA–positive cells).
Tubulointerstitial injury is an important component in the assessment of renal damage because it correlates well with the decline in renal function and long-term progression32,33. In addition, tubular cell injury as a result of chronic ischemia and/or filtered plasma proteins could be crucial in the process of tubulointerstitial fibrosis13.
Many potentially fibrogenic factors, including TGF-, have been implicated in tubulointerstitial injury; however, the role of growth factors in the progression of tubulointerstitial fibrosis remains poorly understood10,34. With respect to the source of fibrogenic growth factors in the kidney, macrophages and mesangial cells have thus far received the most attention.
Myofibroblasts are terminally differentiated cells with morphologic features intermediate between those of fibroblasts and smooth muscle cells. Thus, the cell retains the biologic properties of fibroblast synthesizing interstitial collagens I and III, and at the same time, it expresses -SMA, characteristic of the vascular smooth muscle cell35,36. Although the origin of the myofibroblast remains unknown, Tang et al have recently shown the important role for the myofibroblasts in the pathogenesis of tubulointerstitial fibrosis by infiltrating the tubulointerstitium and as the source of
1(III) collagen36. On the other hand, TGF-
17 and mainly PDGF-BB18,37 were shown to induce transformation of fibroblasts to myofibroblasts, which are major contributors to matrix deposition in the tubulointerstitial area.
Moreover, recently Ito et al have reported a local increase of connective tissue growth factor (CTGF) in human renal fibrosis38. This novel growth factor is strongly up-regulated by TGF- and is coexpressed in
-SMA cells in the tubulointerstitial area. Finally, extracellular matrix production and/or deposition could also be influenced by the presence of MCP-1, either directly as a fibrogenic factor, or indirectly through TGF-
, thus contributing to the development of fibrosis24.
In our patients, the presence of myofibroblasts within the renal interstitium correlates with the extent of tubulointerstitial scarring and functional outcome, confirming other clinical39,40,41 and experimental studies42,43, postulating that the myofibroblasts proliferation can be detected before the presence of significant fibrosis, and suggesting that they may be potentially used as early markers of progressive disease.
In conclusion, our results show that MCP-1, RANTES, and OPN are overexpressed in tubular epithelial cells of patients with more severe and progressive IMN, and that they could participate in the interstitial inflammatory reaction. Based on the experimental observations, we suggest that severe proteinuria is the main factor responsible for the up-regulation of these factors in tubular epithelial cells. The profibrogenic cytokines PDGF-BB and TGF- are also up-regulated in tubular epithelial cells, mainly in the patients with progressive disease, and both growth factors could modulate the interstitial myofibroblastic activity and be potential predictors of progression in IMN.
References
REFERENCES
- Remuzzi, G, Ruggenenti, P, Begnini, A: Understanding the nature of renal disease progression. Kidney Int 1997 51: 2–15, | Article | PubMed | ISI | ChemPort |
- Gansevoort, RT, Navis, GJ, Wapstra, FH, De Jong, PE, De Zeeuw, D: Proteinuria and progression of renal disease: Therapeutic implications. Curr Opin Nephrol Hypertens 1997 6: 133–140, | PubMed | ISI | ChemPort |
- Jungers, P, Hannedouche, T, Itakura, Y, Albouze, G, Descamps-Latscha, B, Mann, NK: Progression rate to end-stage renal failure in non diabetic kidney diseases: A multivariate analysis of determinant factors. Nephrol Dial Transplant 1995 10: 1353–1360, | PubMed | ISI | ChemPort |
- The GISEN Group (Gruppo Italiano Di Studi Epidemiologici In Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non diabetic nephropathy. Lancet 1997 349: 1857–1863, 10.1016/s0140-6736(96)11445-8 | Article | PubMed | ISI
- Bohle, A, Wehrmann, M, MacKensen-Haen, S, Gise, HV, Mick-Eler, E, Xiao, TC, Muller, C, Muller, GA: Pathogenesis of chronic renal failure in primary glomerulonephritis. Nephrol Dial Transplant S 1994 3: 4–12,
- Wehrmann, M, Bohle, A, Bogenschutz, O, Eissele, R, Freisle-Derer, A, Ohlschlegel, C, Schumm, G, Batz, C, Gartner, H-V: Long term prognosis of chronic idiopathic membranous glomerulonephritis. Clin Nephrol 1989 31: 67–76, | PubMed | ISI | ChemPort |
- MacKensen-Haen, R, Eissele, R, Bohle, A: Contribution on the correlation between morphometric parameters gained from the renal cortex and renal function in IgA nephritis. Lab Invest 1988 59: 239–244, | PubMed |
- MacKensen-Haen, S, Bohle, A, Christensen, J, Wehrmann, M, Kendziorra, H, Kokot, F: The consequences for renal function of widening of the interstitium and changes in the tubular epithelium of renal cortex and outer medulla in various renal diseases. Clin Nephrol 1992 37: 70–77, | PubMed | ChemPort |
- Nath, KA: Tubulointerstitial disease as a major determinant of progressive renal injury. Am J Kidney Dis 1992 20: 1–17, | PubMed | ISI | ChemPort |
- Eddy, AA, Giachelli, CM, McCulloch, L, Liu, E: Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int 1995 47: 1546–1557, | Article | PubMed | ISI | ChemPort |
- Wang, Y, Chen, J, Chen, L, Tay, Y-C, Rangan, GK, Harris, DCH: Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol 1997 8: 1537–1545, | PubMed | ISI | ChemPort |
- Abbate, M, Zoja, C, Corna, D, Capitano, M, Bertani, T, Remuzzi, G: In progressive nephropathies, overload of tubular cells with filtered proteins translates glomerular permeability dysfunction into cellular signals of interstitial inflammation. J Am Soc Nephrol 1998 9: 1213–1224, | PubMed | ISI | ChemPort |
- Ong, AC, Fine, LG: Tubular-derived growth factors and cytokines in the pathogenesis of tubulointerstitial fibrosis: Implications for human renal disease progression. Am J Kidney Dis 1994 23: 205–209, | PubMed | ISI | ChemPort |
- Eddy, AA, McCulloch, L, Adams, J, Liu, E: Interstitial nephritis induced by protein-overload proteinuria. Am J Pathol 1989 135: 719–733, | PubMed | ISI | ChemPort |
- Heeger, P, Wolf, G, Meyers, C, Sun, MJ, O'farrell, SC, Krensky, AM, Neilson, EG: Isolation and characterization of cDNA from renal tubular epithelium encoding murine RANTES. Kidney Int 1992 41: 220–225, | Article | PubMed | ISI | ChemPort |
- Border, WA, Noble, NA: TGF-
in kidney fibrosis: A target for gene therapy. Kidney Int 1997 51: 1388–1396, | Article | PubMed | ISI | ChemPort |
- Desmoulière, A, Geinoz, A, Gabbiani, F, Gabbiani, G: Transforming growth factor-
1 induces
-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993 122: 103–111, | Article | PubMed | ISI | ChemPort |
- Tang, WW, Ulich, TR, Lacey, DL, Hill, DC, Qi, M, Kaufman, SA, Van Gy Tarpley, JE, Yee, JS: Platelet-derived growth factor-BB induces renal tubulointerstitial myofibroblast formation and tubulointerstitial fibrosis. Am J Pathol 1996 148: 1169–1180, | PubMed | ISI | ChemPort |
- Okada, H, Strutz, F, Danoff, TM, Neilson, EG: Possible pathogenesis of renal fibrosis. Kidney Int 1996 49 (Suppl 54): S37–S38, | ISI |
- Wasserstein, AG: Membranous glomerulonephritis. J Am Soc Nephrol 1997 8: 664–674, | PubMed | ISI | ChemPort |
- Reichert, LJ, Koene, RA, Wetzels, JF: Prognostic factors in idiopathic membranous nephropathy. Am J Kidney Dis 1998 31: 1–11, | Article | PubMed | ISI | ChemPort |
- Grandaliano, G, Gesualdo, L, Ranieri, E, Monno, R, Montinaro, V, Marra, F, Schena, FP: Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: A pathogenetic role in interstitial monocytes recruitment. J Am Soc Nephrol 1996 7: 906–913, | PubMed | ISI | ChemPort |
- Pichler, R, Giachelli, CM, Lombardi, D, Pippin, J, Gordon, K, Alpers, CE, Schwartz, SM, Johnson, RJ: Tubulointerstitial disease in glomerulonephritis: Potential role of osteopontin (uropontin). Am J Pathol 1994 144: 915–926, | PubMed | ISI | ChemPort |
- Lloyd, C, Gutierrez-Ramos, JC: The role of chemokines in tissue inflammation and autoimmunity in renal diseases. Curr Opin Nephrol Hypertens 1998 7: 281–287, | PubMed | ISI | ChemPort |
- Giachelli, CM, Schwartz, SM, Liaw, L: Molecular and cellular biology of osteopontin: Potential role in cardiovascular disease. Trends Cardiovasc Med 1995 5: 88–95, 10.1016/1050-1738(95)00005-t | Article | ISI | ChemPort |
- Schlondorff, D, Nelson, PJ, Luckow, B, Banas, B: Chemokines and renal disease. Kidney Int 1997 51: 610–621, | Article | PubMed | ISI | ChemPort |
- Gesualdo, L, Grandaliano, G, Ranieri, E, Monno, R, Montinaro, V, Manno, C, Schena, FP: Monocyte recruitment in cryoglobulinemic membranoproliferative glomerulonephritis: A pathogenic role for monocyte chemotactic peptide-1. Kidney Int 1997 51: 155–163, | Article | PubMed | ISI | ChemPort |
- Prodjosudjadi, W, Gerritsma, JS, Vanes, LA, Daha, MR, Brujin, JA: Monocyte chemoattractant protein-1 in normal and diseased human kidneys: An immunohistochemical analysis. Clin Nephrol 1995 44: 148–155, | PubMed | ISI | ChemPort |
- Wada, T, Yokoyama, H, Su, S-B, Mukaida, N, Iwano, M, Dohi, K, Takahashi, Y, Sasaki, T, Furuichi, K, Segawa, C, Hisada, I, Ohta, S, Takasawa, K, Kobayashi, K-I, Matsushima, K: Monitoring urinary levels of monocyte chemotactic and activating factor reflects disease activity of lupus nephritis. Kidney Int 1996 49: 761–767, | Article | PubMed | ISI | ChemPort |
- Cockwell, P, Howie, AJ, Adu, D, Savage, CO: In situ analysis of C-C chemokine mRNA in human glomerulonephritis. Kidney Int 1998 54: 827–836, | Article | PubMed | ISI | ChemPort |
- Remuzzi, G, Zoja, C, Gagliardini, E, Corna, D, Abbate, M, Begnini, A: Combining an antiproteinuric approach with mycophenolate mofetil fully suppresses progressive nephropathy of experimental animals. J Am Soc Nephrol 1999 10: 1542–1549, | PubMed | ISI | ChemPort |
- Risdon, RA, Sloper, JC, De Wardener, HE: Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 1968 2: 363–366, | Article | PubMed | ISI | ChemPort |
- Schainuck, LI, Stricker, GE, Cutler, RE, Benditt, EP: Structural-functional correlations in renal disease. II. The correlations. Hum Pathol 1970 1: 631–641, | Article | PubMed | ChemPort |
- Yamamoto, T, Noble, NA, Miller, DE, Border, WA: Sustained expression of TGF-
1 underlies development of progressive kidney fibrosis. Kidney Int 1994 45: 916–927, | Article | PubMed | ISI | ChemPort |
- Gabbiani, G: The biology of the myofibroblast. Kidney Int 1992 41: 530–532, | Article | PubMed | ISI | ChemPort |
- Tang, WW, Van Gy, QIM: Myofibroblast and
1 (III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int 1997 51: 926–931, | Article | PubMed | ISI | ChemPort |
- Stein-Oakley, AN, Maguire, JA, Dowling, J, Perry, G, Thomson, NM: Altered expression of fibrogenic growth factors in IgA nephropathy and focal and segmental glomerulosclerosis. Kidney Int 1997 51: 195–204, | Article | PubMed | ChemPort |
- Ito, J, Aten, J, Bende, RJ, Oemar, BS, Rabelink, TJ, Weening, JJ, Glodschmeding, R: Expression of connective tissue growth factor in human renal fibrosis. Kidney Int 1998 53: 853–861, | Article | PubMed | ISI
- Roberts, IS, Burrows, C, Shanks, JH, Venning, M, McWilliam, LJ: Interstitial myofibroblasts: Predictors of progression in membranous nephropathy. J Clin Pathol 1997 50: 123–127, | PubMed | ISI | ChemPort |
- Alpers, CE, Hudkins, KL, Floege, J, Johnson, RJ: Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol 1994 5: 201–210, | PubMed | ISI | ChemPort |
- Goumenos, DS, Brown, CB, Shortland, JEL, Nahas, AM: Myofibroblasts, predictors of progression of mesangial IgA nephropathy? Nephrol Dial Transplant 1994 9: 1418–1425, | PubMed | ISI | ChemPort |
- Kliem, V, Johnson, RJ, Alpers, CE, Yoshimura, A, Couser, WG, Koch, KM, Floege, J: Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int 1996 49: 666–678, | Article | PubMed | ISI | ChemPort |
- Diamond, JR, Van Goor, H, Ding, G, Engelmyer, E: Myofibroblasts in experimental hydronephrosis. Am J Pathol 1995 146: 121–129, | PubMed | ISI | ChemPort |
Acknowledgments
This work was supported by research grant from Fondo Nacional de Investigación Científica y Tecnológica, Chile (FONDECYT 1970628) and received support from the Dirección de Investigación, Universidad Austral, Valdivia, Chile. Reproduction of the color figures was supported in part by Roche Chile. A portion of this study was presented at the 31st Annual Meeting of the American Society of Nephrology, Philadelphia, October 1998.
Main navigation
ISN resources
- Nephrology Gateway
- Chinese edition
- Spanish edition
- Portuguese edition
- Contact us
- Society news
NPG resources
- KI Japanese home page
- Nature Reviews Nephrology
- Nature Reviews Urology
NPG Journals
by Subject Area
-
Chemistry
- Chemistry
- Drug discovery
- Biotechnology
- Materials
- Methods & Protocols
Clinical Practice & Research
- Cancer
- Cardiovascular medicine
- Dentistry
- Endocrinology
- Gastroenterology & Hepatology
- Methods & Protocols
- Pathology & Pathobiology
- Urology
Earth & Environment
- Earth sciences
- Evolution & Ecology
Life sciences
- Biotechnology
- Cancer
- Development
- Drug discovery
- Evolution & Ecology
- Genetics
- Immunology
- Medical research
- Methods & Protocols
- Microbiology
- Molecular cell biology
- Neuroscience
- Pharmacology
- Systems biology
Physical sciences
- Physics
- Materials
by A - Z Index
Extra navigation
.ARTICLE NAVIGATION - FULL TEXT
Previous | Next- Table of contents
- Download PDF
- Send to a friend
- Order Commercial Reprints
- CrossRef lists 33 articles citing this article
- Scopus lists 74 articles citing this article
- Save this link
- Abstract
- METHODS
- RESULTS
- DISCUSSION
- References
- Acknowledgments
- Figures and Tables
- Export citation
- Export references
- Papers by Mezzano
- partner of AGORA, HINARI, OARE, INASP, CrossRef and COUNTER